MLD is the sole owner of patents for measuring GTA-446 and other gastric tract acids as a risk factor for pancreatic, colorectal and other cancers.
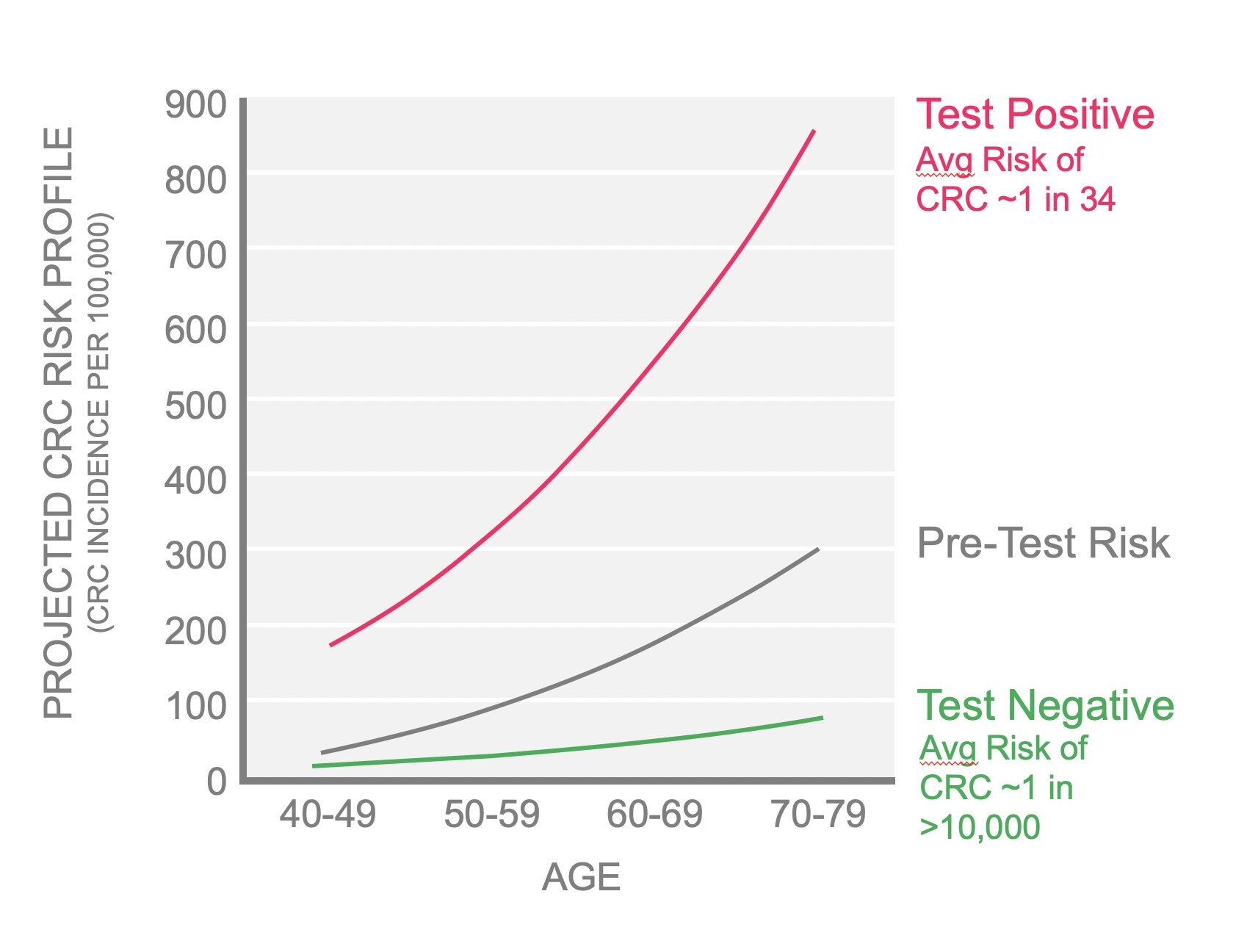
The GTA-446 Test Kit identifies increased risk by splitting the general population into two groups: one with a higher than average incidence of colorectal cancer (red line) and one with a lower than average incidence (green line). Current incidence is shown by the grey line. The difference in the incidences between test-positive and test-negative cohorts (black arrow) is up to 100-fold depending on age. This means a person with a positive test has up to 100-times higher risk than a person with a negative test.
The GTA-446 Test Kit assesses colorectal cancer risk by measuring the levels of GTA-446 in the blood. No bowel preparation, no fasting, and no fecal collection is required. It is a stand-alone kit that can be run on any standard triple-quadrupole mass spectrometer. Each kit processes 90 samples and contains a pre-aliquoted and dried-down standard curve, a stable isotope-labeled internal standard, and complete user manual. The kits are manufactured under stringent quality guidelines.
GTA-446 is a newly discovered long-chain fatty acid present in the body that suppresses inflammation and induces apoptosis. Continued exposure to low levels of GTA-446 over a long period of time could contribute to a chronic pro inflammatory state, and eventually cancer.
If GTA-446 levels are below normal, the test is positive, and the risk of colorectal cancer is increased to approximately 1 in 34. On average, this is up to 50-fold higher compared to a person with a negative test result*. In comparison, the increased risk associated with family history (a first degree family member diagnosed) is only 1.5-fold.
People with positive test results should adhere to current colorectal cancer screening guidelines. People with a negative result should be retested every two years. A physician should review all test results and be consulted to help manage individual risk.
The GTA-446 Test Kit is intended for use in assessing risk and monitoring; it is not a standalone diagnostic test, and is not a screening test for colon cancer.
The GTA-446 metabolite is a fatty acid involved in protecting against chronic inflammation through the downregulation of NFκB. When levels of GTA-446 become deficient (positive result, right panel) it can no longer block NFκB expression resulting in the induction of multiple proinflammatory proteins. This creates an oxidative environment in the gastrointestinal tract that can lead to DNA mutations in cells, and ultimately increased cancer risk. GTA-446 is therefore not a tumor marker like occult blood or methylated DNA; rather, it is a pre-disease metabolic deficiency that results in a pro-cancer environment within the body. It is currently hypothesized that GTA-446 is a metabolite linked to the gut microbiome.
References
Downregulation of serum metabolite GTA-446 as a novel potential marker for early detection of colorectal cancer. British Journal of Cancer. 117, 2017.
Reduced levels of hydroxylated, polyunsaturated ultra long-chain fatty acids in the serum of colorectal cancer patients: implications for early screening and detection. BMC Medicine. Feb 2010; 8: 13.
Reduction of novel circulating long-chain fatty acids in colorectal cancer patients is independent of tumor burden and correlates with age. BMC Gastroenterology. Nov. 2010; 10:140.
Human serum-derived hydroxyl long-chain fatty acids exhibit antiinflammatory and anti- proliferative activity. Journal of Experimental & Clinical Cancer Research. 30:59, 2011
Low-serum GTA-446 anti-inflammatory fatty acid levels as a new risk factor for colon cancer. Int J Cancer. Jan 15, 2013; 132(2):355-62.
*Based on the difference in predicted CRC incidence rates between subjects with GTA-446 positive and negative test results and Canadian Cancer Statistics 2012.